Chemistry Formula List
Introduction
Whether you are a student taking JC Chemistry, O-Level Chemistry or Secondary School Science, Chemistry Formulas are important are often used and referred to during Examinations. It is necessary for students to be familiar with the terms, formulas, symbols, and equations used.
Sophia Education provides you a simplistic formula list and guide for our students to refer to.

What is a Chemical Formula?
A chemical formula is an expression that states the number and type of atoms present in a molecule of a substance. The type of atom is given using element symbols. The number of atoms is indicated by a subscript following the element symbol.

Types of Chemical Formulas
While any expression that cites the number and kind of atoms is a chemical formula, there are different types of formulas, including molecular, empirical, structure, and condensed chemical formulas.



Download Our
Chemistry Formulas List Below

Citations
Helmenstine, Anne Marie, Ph.D. “What Is a Chemical Formula?” ThoughtCo, Sep. 1, 2021, thoughtco.com/definition-of-chemical-formula-604906.
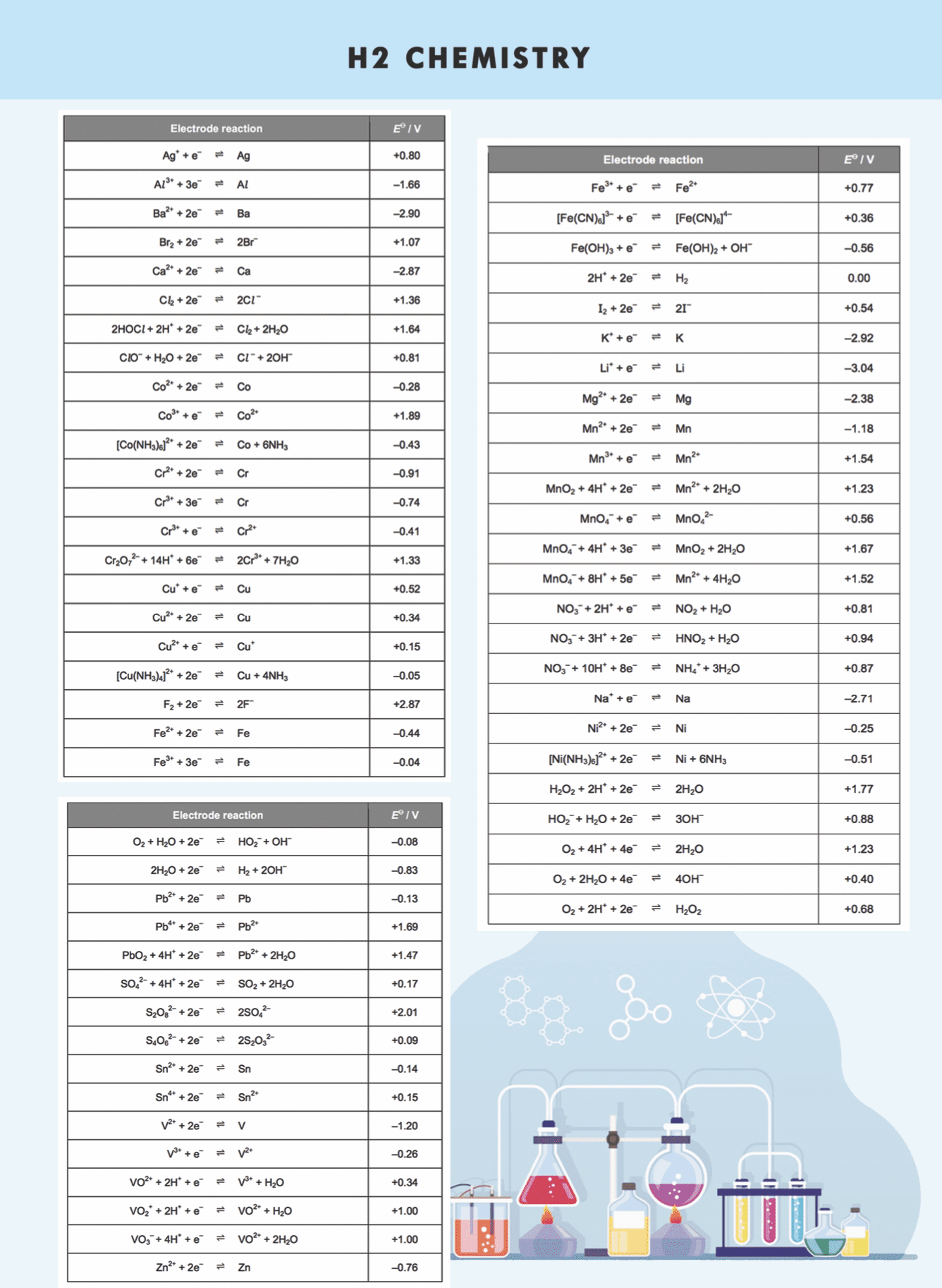
Basic Physical Chemistry Formulae List @2018 Mr Chong Chemistry Tuition. chemistry formulas, chemistry formulas in singapore, Best chemistry formulas in singapore
For Cambridge-Singapore A levels H2 Chemistry 9729
chemistry formulas, chemistry formulas in singapore, Best chemistry formulas in singapore
- Atoms, Molecules and Stoichiometry
Amount (in moles) = mass (g) / molar mass (g mol-1)
n = m / M
Amount of a gas = volume (dm3) / molar volume (dm3)
Amount = number of particles (ions, molecules, atoms) / Avogadro constant n = N / L
At s.t.p. (standard temperature and pressure of 1 bar and 273 K), molar volume of a gas is 22.7 dm3.
At r.t.p. (room temperature and pressure of 1 atm and 293 K), molar volume is 24.0 dm3. Limiting reagent is the reactant that is not in excess and is completely used up in the reaction. Concentration in g dm-3 = mass of solute (g) / volume of solution (dm3) Concentration in mol dm-3 = Amount of solute (mol) / volume of solution (dm3) Concentration in g dm-3 = concentration in mol dm-3 x Molar mass (g)
In dilution,
Amount of solute in original solution = Amount of solute in dilute solution C original conc x V original vol. = C diluted conc. x V diluted vol.
Combustion of a hydrocarbon:
CxHy + (x + y/4)O2 🡪 xCO2 + (y/2)H2O
- Gases
pV = nRT
R : gas constant (8.31 J K-1 mol-1)
P : pressure in Pa
V : volume in m3
T : Temperature in Kelvins
- Chemical Bonding
Ionic Bond strength α |��+ ∗ ��−|
|��+|+|��−|q : charge r: ionic radius
2
@2018 Mr Chong Chemistry Tuition. Whatsapp 98935144. http://www.alevelchemistrysg.com
- Energetics
Q = mc∆T
Q : Heat change
M : mass
c : specific heat capacity
∆T : change in temperature
∆H = ± ����
∆H : Enthalpy change
Q : Heat change
n : Amount (mol)
∆H = ∑Hc (reactants) – ∑Hc (products)
∆Hsolution = ∆Hhydration – Lattice Energy
∆GƟ = ∆HƟ – T∆SƟ
∆GƟ : Standard Gibbs Free Energy
∆SƟ : Entropy change
If ∆GƟ < 0, reaction is spontaneous. chemistry formulas, chemistry formulas in singapore, Best chemistry formulas in singapore
If ∆GƟ = 0, reaction is in equilibrium.
If ∆GƟ > 0, reaction is non-spontaneous.
- Kinetics
A + B 🡪 C + D
Rate = k[A]m[B]n
m, n : orders of reaction with respect to reactants A and B k : rate constant
3
@2018 Mr Chong Chemistry Tuition. Whatsapp 98935144. http://www.alevelchemistrysg.com
First order reaction
Half life, t1/2 = ln 2 / k
Half life is constant for a first order reaction.
- Chemical equilibrium
aA + bB cC + dD
Kc = ([C]c[D]d) / ([A]a[B]b)
Kc = equilibrium constant
[X] = concentration of X in mol dm-3
For a system involving gases,
aA (g) + bB (g) cC (g) + dD (g)
Kp = Pcc PDd/ PAa PBb
Kp = equilibrium constant expressed in terms of equilibrium partial pressures Px = partial pressure of X in atm, bar or Pa
Kc = kforward reaction / kbackward reaction
∆GƟ = -RT ln K
K : equilibrium constant
- Ionic equilibria
pH = – log [H+]
Consider a weak monoprotic acid, HA, which dissociates partially in water : HA (aq) + H2O (l) H3O+(aq) + A–(aq)
At equilibrium,
+ −
Ka = [ ][ ]
H A
[HA]
[H+] = √Ka [HA]
4
@2018 Mr Chong Chemistry Tuition. Whatsapp 98935144. http://www.alevelchemistrysg.com
Ka : acid equilibrium constant
Base dissociation constant, Kb. B + H2O BH+ + OH
HB OH + −
Kb = [ ][ ]
[B]
B : weak base
Kw = [H+][OH–]
Kw : Ionic Product of water
pKw = pH + pOH
= 14 at 25 0C
Buffers
Acidic buffer:
pH = pKa + log [salt]/[acid]
Alkaline buffer: chemistry formulas, chemistry formulas in singapore, Best chemistry formulas in singapore
pOH = pKb + log10[ ]
Salt
[Base]
Solubility Equilibria
aA(s)⇌cC(aq)+dD(aq)
Ksp=[C]c[D]d
Solubility product of a sparingly soluble salt, A : Ksp
8.Electrochemistry
EcellƟ = EreductionƟ – EoxidationƟ
EcellƟ : standard cell potential
Anode is where oxidation takes place; cathode is where reduction takes place. EcellƟ > 0, reaction is feasible
5
@2018 Mr Chong Chemistry Tuition. Whatsapp 98935144. http://www.alevelchemistrysg.com
∆GƟ = -nFEcellƟ
n : amount of electrons transferred in the electrochemical reaction F : Faraday constant (96500 C mol-1)
Q = It
Q = neF
Q : amount of charge in C
I : current
t : time
ne : amount of electrons
EcellƟ = ����
����ln K
K : equilibrium constant
n : amount of electrons transferred in the electrochemical reaction Mass of substance liberated in electrolysis α Q
F = Le
L : Avogadro constant
e : charge on one electron (1.60 x 10-19 C)

