Pure Chemistry Sec 3/4 Notes By Sophia Education
Sophia Education’s Chemistry Notes help you to save 60% of your study time. Get the most effective and comprehensive revision material covering all syllabus topics, as well as answers to every question on the practice model papers
Pure Chemistry
Sophia Education’s best teachers and tutors have compiled the most efficient and concise notes specially for our students! Our notes are straight to the point with only necessary points to help to improve and be ahead of your peers. The pure chemistry notes are for elementary to advanced students specifically who want pure chemistry revision or pure chemistry homework help.
Our pure chemistry notes contain very little fluff and only the necessary points you need to know for pure chemistry classes. The pure chemistry notes are designed to improve your grades in pure chemistry classes by helping our students study efficiently, step-by-step with concise explanations.
PURE CHEMISTRY NOTES SUMMARY
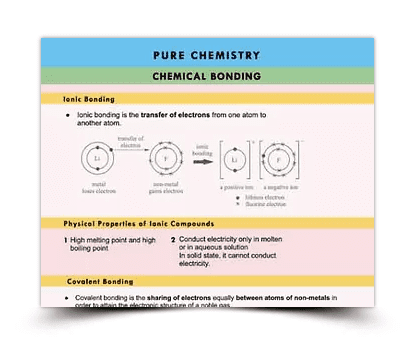
Summary Notes
Learn Chemistry the easy way with Sophia Education
PURE CHEMISTRY FORMULA LIST
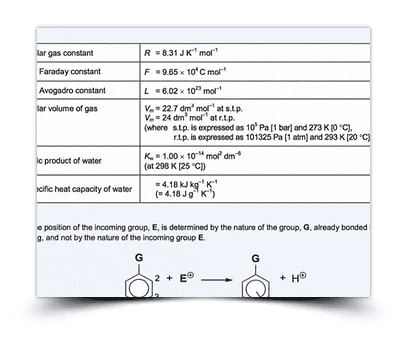
Formula List
Chemistry formulas you need to know for Sec 3/4 Pure Chemistry
SIGN UP FOR OUR CHEMISTRY TUITION
Do you need a little Chemistry help? Our CHemistry tuition and notes will give you the boost you need to improve your grades in just 12 weeks! Concise and easy-to-follow, our tuition is perfect for students of all levels.
What Is Pure Chemistry?
Have you ever wondered why water freezes or how nylon is made? Do you like to figure things out just for fun, even if you can’t necessarily use the information for anything? You might enjoy pure chemistry.
If we step into the field of chemistry we will see there are two broad categories: applied chemistry . You can think of each branch as sisters. Although they are related, they have very unique differences. Chemistry is the ability to study something for your own knowledge benefit. On the other hand, applied chemistry is the process of using your knowledge for an intended purpose or application.
Let’s think of it this way, pure chemistry is our theoretical brain of chemistry. This simply means that, as a pure chemist, you like to investigate the theory or principles of important chemistry topics. Applied chemistry is our practical brain of chemistry. As an applied chemist, you research the different ways to solve real world problems.
But wait a minute; did you know chemistry could help its sister, applied chemistry? Yep, it can! Findings from pure chemistry research can provide a great stepping stone for applied chemistry research. There are so many projects created from scientists who were curious about something, which in the end, led to useful discoveries.
Wilhelm Rontgen and his work lead to the X-ray
x ray
Meet Wilhelm Röntgen. He was a scientist truly curious about these things called ‘crookes tubes.’ Learning all he could about these tubes eventually led to the discovery of an X-ray. We can take his initial work and categorize it as a form of chemistry research. However, the ability to take that work and apply it to the production of a useful product, the X-ray, is what we call applied chemistry.
Save Timeline Autoplay
Video Quiz Course
22K views
Chemistry at Work: Examples
There are several examples that involved the use of pure chemistry. Our first example involves materials we can find in a variety of linen products. These materials are cotton and silk. Many, many years ago, a scientist by the name of Hermann Staudinger, wanted to learn more about the molecular structure of cotton and silk fibers.
Appropriate Creator
We have already talked about valence electrons and how valence electrons are important in determining the chemical reactions of different types of atoms. As a reminder about an atom that has an incomplete valence shell – for example, if it is an atom that has an electron in the first shell, the atom is not stable and wants to do what it can to stabilize its energy. . There are basically three different ways an atom can try to create a complete valence level:
1) It may try to add electrons.
2) It may try to give away electrons.
3) It may decide to share electrons.
This sharing strategy is what we are going to talk about today.
Save timeline autoplay speed to normal
Video quiz course
56 thousand views
Creating a molecule
When one atom comes in contact with another atom, it forms what is called a chemical bond. This interaction, this chemical bond, binds two atoms together into something called a molecule.
To form a covalent bond
Now, we are going to talk about covalent bonds, which means that atoms are going to share electrons. Covalent bonds are very strong bonds. These are very important in biology because they are very stable and because most biological molecules are made up of covalent bonds. These biological molecules are then very stable.
So, let’s take an atom with multiple valence electrons – for example carbon. Carbon has four valence electrons, so it has the potential to form four different covalent bonds.